9 Horrifically Botched Clinical Trials
Nathan Johnson
Published
03/28/2017
in
wtf
Some people volunteer for medical testing as a way to make a little extra cash, while others are unwitting participants. But it's not always the safest bet.
- List View
- Player View
- Grid View
Advertisement
-
1.
 In the mid-'50s, Thalidomide was marketed as a sedative that was safe to use “even during pregnancy,” as its developers “could not find a dose high enough to kill a rat.” By 1960, it was available in 46 countries, with sales matching those of aspirin. An Australian obstetrician, Dr. William McBride, discovered that the drug alleviated morning sickness and recommended it to his pregnant patients, starting a worldwide trend. But by 1961, he began to associate the so-called harmless drug with severe birth defects in the babies he delivered. Many of were born with phocomelia, resulting in shortened, absent, or flipper-like limbs. Within a year, Thalidomide was banned in most countries around the world. The tragedy surrounding the drug helped motivate profound changes in the Food and Drug Administration (FDA). By passing the Kefauver-Harris Drug Amendments Act in 1962, legislators tightened restrictions on drug testing in the U.S., requiring that manufacturers prove what they're selling is both safe and effective.
In the mid-'50s, Thalidomide was marketed as a sedative that was safe to use “even during pregnancy,” as its developers “could not find a dose high enough to kill a rat.” By 1960, it was available in 46 countries, with sales matching those of aspirin. An Australian obstetrician, Dr. William McBride, discovered that the drug alleviated morning sickness and recommended it to his pregnant patients, starting a worldwide trend. But by 1961, he began to associate the so-called harmless drug with severe birth defects in the babies he delivered. Many of were born with phocomelia, resulting in shortened, absent, or flipper-like limbs. Within a year, Thalidomide was banned in most countries around the world. The tragedy surrounding the drug helped motivate profound changes in the Food and Drug Administration (FDA). By passing the Kefauver-Harris Drug Amendments Act in 1962, legislators tightened restrictions on drug testing in the U.S., requiring that manufacturers prove what they're selling is both safe and effective. -
2.
 In 1953, a young RAF airman, Ronald Maddison, died after being exposed to sarin at Porton Down in Wiltshire. In the study, Maddison was told he was helping to find a cure for the common cold. He was offered 15 shillings and a three-day leave pass. He planned to use the money to purchase an engagement ring for his girlfriend, Mary Pyle. Once in the facility, he was exposed to 200 milligrams of sarin nerve gas which was dropped onto a piece of uniform material wrapped around his arm. Within twenty minutes, he began to sweat and complained that he did not feel well. He soon slumped over the table. A few moments later, he complained of deafness and started gasping for breath and convulsing. He was taken to a nearby medical facility, where doctors attempted to resuscitate him before injecting adrenaline into his heart. Nothing worked.Less than 45 minutes after being exposed to the poison, he was pronounced dead.
In 1953, a young RAF airman, Ronald Maddison, died after being exposed to sarin at Porton Down in Wiltshire. In the study, Maddison was told he was helping to find a cure for the common cold. He was offered 15 shillings and a three-day leave pass. He planned to use the money to purchase an engagement ring for his girlfriend, Mary Pyle. Once in the facility, he was exposed to 200 milligrams of sarin nerve gas which was dropped onto a piece of uniform material wrapped around his arm. Within twenty minutes, he began to sweat and complained that he did not feel well. He soon slumped over the table. A few moments later, he complained of deafness and started gasping for breath and convulsing. He was taken to a nearby medical facility, where doctors attempted to resuscitate him before injecting adrenaline into his heart. Nothing worked.Less than 45 minutes after being exposed to the poison, he was pronounced dead. -
3.
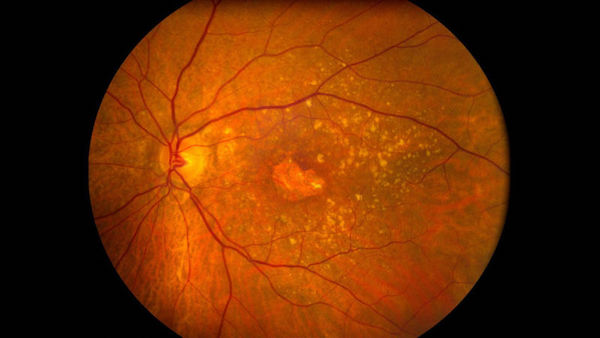 A questionable stem cell therapy clinical trial conducted by a Florida clinic has blinded three patients permanently. The women, from 72 to 88 years old, were suffering from macular degeneration, a common progressive disease of the retina. They were experiencing various degrees of vision loss and sought help for which they paid $5000. A week after stem cells were injected into their eyes (yes, both eyes, a big no-no in the field of ophthalmological research), the women went blind, and doctors say there's virtually no chance their vision will be restored. Under the right conditions, stem cells can be transformed into almost any kind of cell found in the body, which is why they've proven useful in regenerative medicine. Scientists hope to be able to use them to repair damaged tissue and organs—and possibly even the effects of macular degeneration—but we're not there yet. Currently, they are only used in bone marrow transplants, but that hasn't stopped nearly 600 clinics nationwide from peddling unproven stem-cell procedures for a broad range of conditions, including arthritis, autism, cerebral palsy, stroke, muscular dystrophy, and cancer.
A questionable stem cell therapy clinical trial conducted by a Florida clinic has blinded three patients permanently. The women, from 72 to 88 years old, were suffering from macular degeneration, a common progressive disease of the retina. They were experiencing various degrees of vision loss and sought help for which they paid $5000. A week after stem cells were injected into their eyes (yes, both eyes, a big no-no in the field of ophthalmological research), the women went blind, and doctors say there's virtually no chance their vision will be restored. Under the right conditions, stem cells can be transformed into almost any kind of cell found in the body, which is why they've proven useful in regenerative medicine. Scientists hope to be able to use them to repair damaged tissue and organs—and possibly even the effects of macular degeneration—but we're not there yet. Currently, they are only used in bone marrow transplants, but that hasn't stopped nearly 600 clinics nationwide from peddling unproven stem-cell procedures for a broad range of conditions, including arthritis, autism, cerebral palsy, stroke, muscular dystrophy, and cancer. -
4.
 When Kano, Nigeria was hit by Africa's worst ever meningitis epidemic in 1996, 11 children died in a clinical trial after being given an experimental oral antibiotic called Trovan. Others received ceftriaxone, the "gold-standard" treatment of modern medicine. Five children died on Trovan and six on ceftriaxone. It was claimed that the drug's creators, Pfizer, did not have proper consent from parents to use an experimental drug on their children. Legal action was filed against the company. It was also alleged that some of the kids received a dose lower than recommended, leaving many with brain damage, paralysis or slurred speech. In 2009, Pfizer reached a tentative, out-of-court settlement with the Kano state government worth $75 million. The families of the deceased children were awarded $175,000 each.
When Kano, Nigeria was hit by Africa's worst ever meningitis epidemic in 1996, 11 children died in a clinical trial after being given an experimental oral antibiotic called Trovan. Others received ceftriaxone, the "gold-standard" treatment of modern medicine. Five children died on Trovan and six on ceftriaxone. It was claimed that the drug's creators, Pfizer, did not have proper consent from parents to use an experimental drug on their children. Legal action was filed against the company. It was also alleged that some of the kids received a dose lower than recommended, leaving many with brain damage, paralysis or slurred speech. In 2009, Pfizer reached a tentative, out-of-court settlement with the Kano state government worth $75 million. The families of the deceased children were awarded $175,000 each. -
5.
 The FDA suspended a clinical trial of a treatment by biopharmaceutical company Juno Therapeutics after two cancer patients died in 2016. A patient in a previous Juno trial died from cerebral edema, a swelling in the brain that is the result of excessive fluid, which also killed the patients in the 2016 trial. The drug company decided that their deaths were due to a confluence of factors, one of which was the addition of another cancer-fighting drug to the treatment regimen. It has since proposed that the FDA continue the trial without using the cancer drug that it had added to the preconditioning regimen.
The FDA suspended a clinical trial of a treatment by biopharmaceutical company Juno Therapeutics after two cancer patients died in 2016. A patient in a previous Juno trial died from cerebral edema, a swelling in the brain that is the result of excessive fluid, which also killed the patients in the 2016 trial. The drug company decided that their deaths were due to a confluence of factors, one of which was the addition of another cancer-fighting drug to the treatment regimen. It has since proposed that the FDA continue the trial without using the cancer drug that it had added to the preconditioning regimen. -
6.
 18-year-old Jesse Gelsinger had a condition called ornithine transcarbamylase deficiency (OTC), but it was under control through a combination of diet and medication. He lacked a functional enzyme involved in breaking down ammonia, a waste product of protein metabolism that becomes toxic when its levels become too high. The disease is fatal in infants, but he had not inherited it. It was, for him, the result of a spontaneous genetic mutation after conception and was not as severe. The otherwise healthy teen joined a clinical trial at the University of Pennsylvania aimed at developing a treatment for infants born with the disease. It was there he was injected with an adenoviral vector carrying a corrected gene to test the safety of the procedure. Four days later he passed away, after having suffered a massive immune response triggered by the use of the viral vector used to transport the gene into his cells, leading to multiple organ failure and brain death. His 1999 death has since slowed progress in gene therapy for any condition.
18-year-old Jesse Gelsinger had a condition called ornithine transcarbamylase deficiency (OTC), but it was under control through a combination of diet and medication. He lacked a functional enzyme involved in breaking down ammonia, a waste product of protein metabolism that becomes toxic when its levels become too high. The disease is fatal in infants, but he had not inherited it. It was, for him, the result of a spontaneous genetic mutation after conception and was not as severe. The otherwise healthy teen joined a clinical trial at the University of Pennsylvania aimed at developing a treatment for infants born with the disease. It was there he was injected with an adenoviral vector carrying a corrected gene to test the safety of the procedure. Four days later he passed away, after having suffered a massive immune response triggered by the use of the viral vector used to transport the gene into his cells, leading to multiple organ failure and brain death. His 1999 death has since slowed progress in gene therapy for any condition. -
7.
 In March 2006, eight men volunteered to try a new drug with hopes of significantly improving cancer treatment by manipulating the immune system. They were each offered £2,000. Soon after taking after taking the TGN1412 drug, six of the eight patients (two were placebos) were writhing and screaming in agony as their organs started to fail and their heads began to swell. Medics battled to save the young men, who were in ICU for several weeks before being released. The youngest participant, Ryan Wilson, then 21, had to spend four months in the hospital after suffering heart, liver and kidney failure. He also experienced frostbite-like effects that led to sections of his feet being amputated and some of his fingertips falling off. The men still don't know what the long-term damage to their bodies will be. The trial was carried out at Northwick Park Hospital in London and was run by Parexel, an American multinational life sciences consulting firm.
In March 2006, eight men volunteered to try a new drug with hopes of significantly improving cancer treatment by manipulating the immune system. They were each offered £2,000. Soon after taking after taking the TGN1412 drug, six of the eight patients (two were placebos) were writhing and screaming in agony as their organs started to fail and their heads began to swell. Medics battled to save the young men, who were in ICU for several weeks before being released. The youngest participant, Ryan Wilson, then 21, had to spend four months in the hospital after suffering heart, liver and kidney failure. He also experienced frostbite-like effects that led to sections of his feet being amputated and some of his fingertips falling off. The men still don't know what the long-term damage to their bodies will be. The trial was carried out at Northwick Park Hospital in London and was run by Parexel, an American multinational life sciences consulting firm. -
8.
 In 2003, Dr. Stephen Olson, an associate professor in the University of Minnesota's Department of Psychiatry, coerced a young, psychotic man into taking part in a AstraZeneca-funded drug study of antipsychotic drugs (most notably Seroquel) aimed at patients experiencing their first psychotic episode—by using the threat of involuntary commitment. Dan Markingson signed up for the "CAFÉ" study. His mother, Mary Weiss, objected to her son's enrollment and tried desperately for months to get him out, believing that his condition was deteriorating and that he was in danger of committing suicide. Her warnings were consistently ignored and Dan continued with the study. Five months after starting, he died after trying to decapitate himself with a box-cutter. Soon after his death, the university suspended recruitment into psychiatric research studies. On April 9, 2015, Charles Schulz, M.D. announced his resignation as Chair of the Department of Psychiatry as a result of the tragedy.
In 2003, Dr. Stephen Olson, an associate professor in the University of Minnesota's Department of Psychiatry, coerced a young, psychotic man into taking part in a AstraZeneca-funded drug study of antipsychotic drugs (most notably Seroquel) aimed at patients experiencing their first psychotic episode—by using the threat of involuntary commitment. Dan Markingson signed up for the "CAFÉ" study. His mother, Mary Weiss, objected to her son's enrollment and tried desperately for months to get him out, believing that his condition was deteriorating and that he was in danger of committing suicide. Her warnings were consistently ignored and Dan continued with the study. Five months after starting, he died after trying to decapitate himself with a box-cutter. Soon after his death, the university suspended recruitment into psychiatric research studies. On April 9, 2015, Charles Schulz, M.D. announced his resignation as Chair of the Department of Psychiatry as a result of the tragedy. -
9.
 In 2016, six men were hospitalized (and one was killed) after participating in a drug trial in northwestern France at the Centre Hospitalier Universitaire de Rennes, in eastern Brittany. Of 128 participants, 90 were given the unnamed drug and the rest were given a placebo. It was intended to help with mood, anxiety, and motor problems linked to neurodegenerative diseases. During Phase 1 of the trial by Biotrial, a drug evaluation company based in Rennes, three of the six affected patients, aged 28 to 49, suffered irreversible brain damage. An investigation is ongoing—it's now claimed that the drug had left “a number” of dogs dead and others with neurological damage before being tested on humans.
In 2016, six men were hospitalized (and one was killed) after participating in a drug trial in northwestern France at the Centre Hospitalier Universitaire de Rennes, in eastern Brittany. Of 128 participants, 90 were given the unnamed drug and the rest were given a placebo. It was intended to help with mood, anxiety, and motor problems linked to neurodegenerative diseases. During Phase 1 of the trial by Biotrial, a drug evaluation company based in Rennes, three of the six affected patients, aged 28 to 49, suffered irreversible brain damage. An investigation is ongoing—it's now claimed that the drug had left “a number” of dogs dead and others with neurological damage before being tested on humans.
In the mid-'50s, Thalidomide was marketed as a sedative that was safe to use “even during pregnancy,” as its developers “could not find a dose high enough to kill a rat.” By 1960, it was available in 46 countries, with sales matching those of aspirin. An Australian obstetrician, Dr. William McBride, discovered that the drug alleviated morning sickness and recommended it to his pregnant patients, starting a worldwide trend. But by 1961, he began to associate the so-called harmless drug with severe birth defects in the babies he delivered. Many of were born with phocomelia, resulting in shortened, absent, or flipper-like limbs. Within a year, Thalidomide was banned in most countries around the world. The tragedy surrounding the drug helped motivate profound changes in the Food and Drug Administration (FDA). By passing the Kefauver-Harris Drug Amendments Act in 1962, legislators tightened restrictions on drug testing in the U.S., requiring that manufacturers prove what they're selling is both safe and effective.
9/9
1/9
Categories:
Wtf









0 Comments